Peptide Modifications
|
FITC
|
Rhodamine B
|
|
|
|

|
|
FITC Conjugated Peptides
|
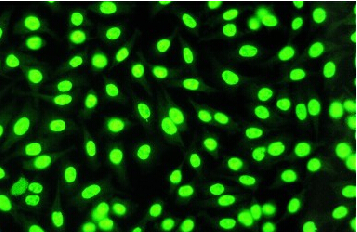
FITC-Peptides Under Microscopy
|
Rhodamine in 3d model
|
Rhodamine B in solution
|
ChinaPeptides offers a wide range of peptide modification services including the follwings:
1. Phosphorylation
Phosphopeptides can assist in the investigation of the influences of phosphorylation on peptides and protein structure and in the understanding of regulatory processes mediated by protein kinases. GenScript has successfully synthesized numerous serine-, threonine-, and tyrosine-phosphopeptides. For peptides containing one or more of these hydroxy-amino acids, selective phosphorylation can be achieved by orthogonal protection or by Fmoc-protected phosphorylated amino acids.
2. MAP Application
Multiple antigen peptide application is one potent way to produce high-titer anti-peptide antibodies and synthetic peptide vaccines. This system utilizes the a- and e-amino groups of lysine to form a backbone to which multiple peptide chains can be attached. Depending on the number of lysine tiers, different numbers of peptide branches can be synthesized. This eliminates the need to conjugate the antigen to a protein carrier.
3. KLH, BSA, and Ovalbumin
Peptide antigens are often too small to generate significant immune responses on their own. To solve this problem, these peptides are conjugated to bigger carrier proteins, such as bovine serum albumin (BSA), ovalbumin, or keyhole limpet hemocyanin (KLH). One of the advantages of KLH is that it does not interfere with ELISA or western blotting because it is not used as a blocking reagent. One common means of conjugation method is the maleimide method, which couples the cysteine residue of the peptide to the carrier protein. To perform this conjugation, one cysteine residue is added to the N- or C-terminus of the peptide so that it may be linked to the carrier protein.
Note: KLH is a large (MW = 4*105 to 1*107) aggregating protein. Because of its size and structure, its solubility in water is often limited, giving solutions and mixtures a cloudy appearance. This does not affect immunogenicity and the turbid solution can be used for immunizations.
4. Amidation and Acetylation
If the peptide is from an internal sequence of a protein, terminal amidation (C-terminus) or acetylation (N-terminus) will remove its charge and help it imitate its natural structure (amide, CONH2). In addition, this modification makes the resulting peptide more stable towards enzymatic degradation resulting from exopeptidases.
5. Biotin and FITC
Fluorescein isothiocyanate (FITC) is an activated precursor used for fluorescein labeling. For efficient N-terminal labeling, a seven-atom aminohexanoyl spacer (NH2-CH2-CH2-CH2-CH2-CH2-COOH) is inserted between the fluorophore (fluoroscein) and the N-terminus of the peptide.
For C-terminal labeling of biotin, a Lys residue is added to the C-terminus of the peptide. Biotin is then attached to the lysine side chain via amide bond. The positive charge of the lysine is then removed.